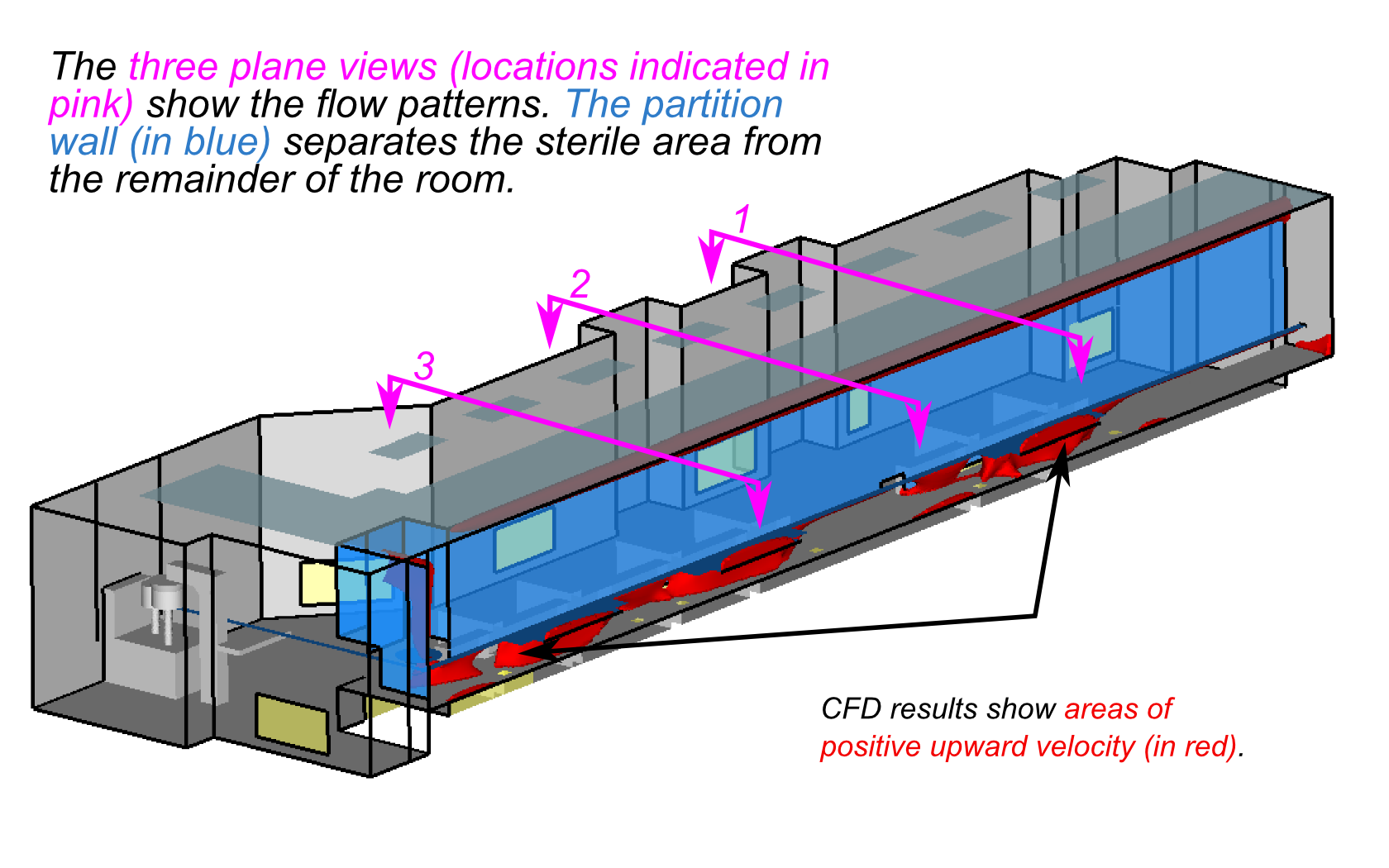
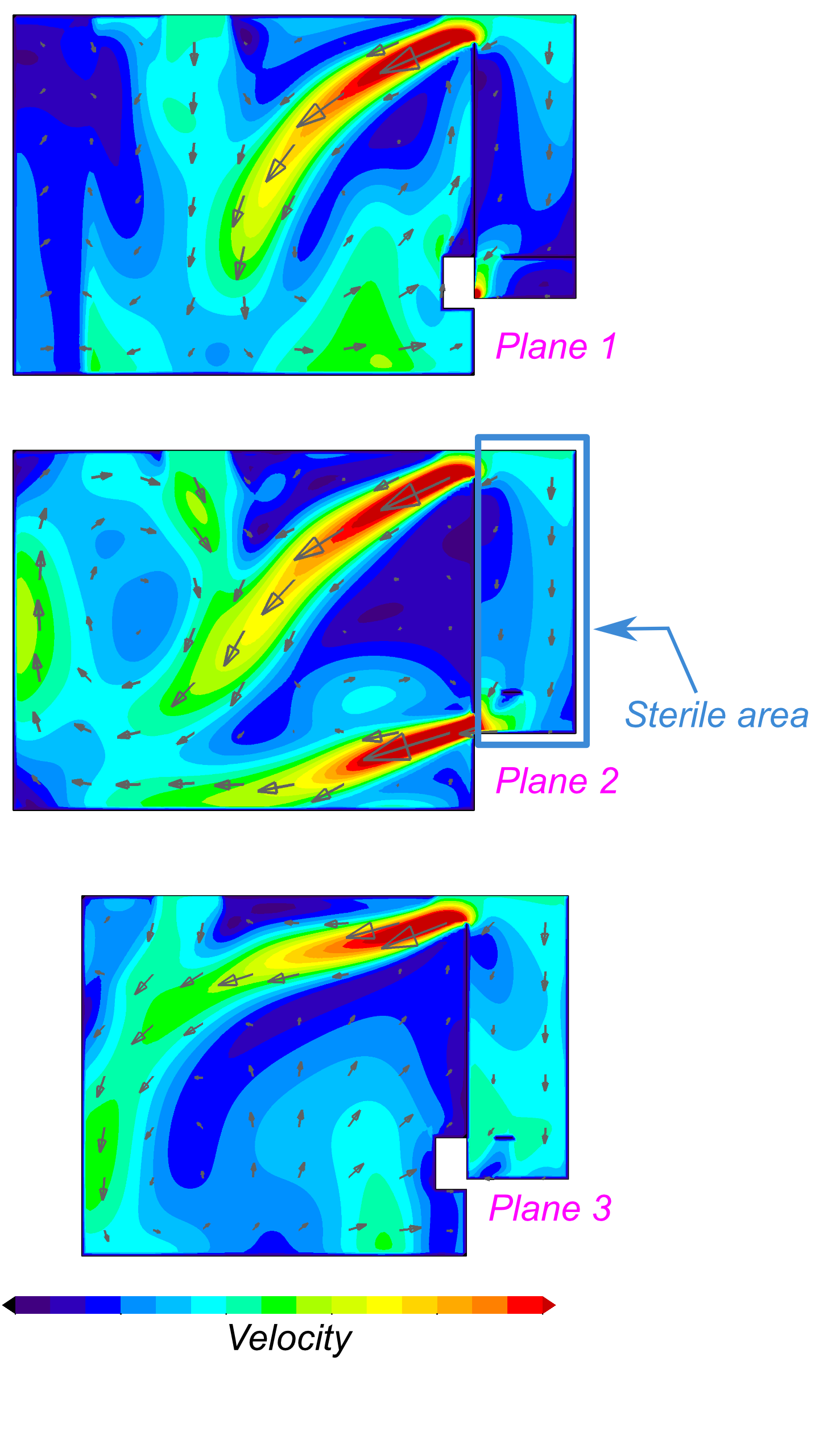
 Clean rooms used in pharmaceutical manufacturing operations use downflow of
Clean rooms used in pharmaceutical manufacturing operations use downflow of
HEPA filtered air to ensure sterility. While experimental methods can be used to
detect regions of upward flow which can potentially transport contaminants up to
the product level, modifications to an existing clean room will affect cost and
start-up schedules. CFD offers an alternative to assess and correct system
design at the concept stage, when those modifications have a smaller effect on
project budget.
The images below show the analysis of a clean room that was slated for
increased capacity, including additional product equipment and revision to the
return air register locations. CFD analyses showed that the proposed changes
did not significantly affect the flow patterns in the critical regions of the clean
room, allowing the modifications to proceed with confidence.